
Staburo at the BVMA symposium 2016 in Munich
 Staburo took part in this year’s BVMA symposium on 25 November 2016, at the Sheraton München Arabellapark Hotel, here in Munich.
Staburo took part in this year’s BVMA symposium on 25 November 2016, at the Sheraton München Arabellapark Hotel, here in Munich.
The BVMA represents the interests of the German CROs. The member companies maintain a lively exchange of information on all topics which are of particular importance to the CRO branch, e.g. on
- Questions of liability and insurance
- Education and training
- Quality requirements and
- Trends in the pharmaceutical and CRO industry
We also attended the get-together on 24 November 2016, at the Sheraton München Arabellapark Hotel, where we met current partners and other companies that currently need biostatistics support.
This year’s sessions gave a great update on regulatory news, risk-based clinical research and advanced study designs. Especially the latter was very interesting for us, since we see an increasing demand from our clients in this field and build up a very good expertise in recent years, e.g. in adaptive/Bayesian designs.
Big thank you to the great organization of this event, despite the interfering Lufthansa strike!

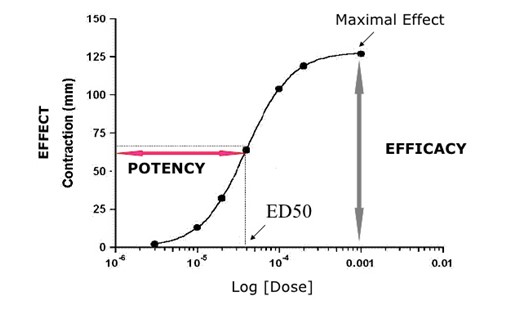
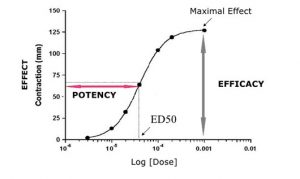 In this talk, important aspects of pharmacokinetics (PK) and pharmacodynamics (PD) were presented. Four elements of PK (ADME) were discussed in detail. Biopharmaceutical classification system (BCS), 2-compartment distribution model, destructive and conjugative reactions and the elimination phase of drugs were discussed for ADME trials. Some PK parameters were also listed and described. The second part of this talk was about pharmacodynamics, where the onset, duration and mechanism of actions were presented. In addition, some PD concepts such as ED50, efficacy and potency were discussed.
In this talk, important aspects of pharmacokinetics (PK) and pharmacodynamics (PD) were presented. Four elements of PK (ADME) were discussed in detail. Biopharmaceutical classification system (BCS), 2-compartment distribution model, destructive and conjugative reactions and the elimination phase of drugs were discussed for ADME trials. Some PK parameters were also listed and described. The second part of this talk was about pharmacodynamics, where the onset, duration and mechanism of actions were presented. In addition, some PD concepts such as ED50, efficacy and potency were discussed.

 Staburo took part in the 2016 BIO Europe Conference in Cologne between 7 and 9 November.
Staburo took part in the 2016 BIO Europe Conference in Cologne between 7 and 9 November.
Recent Comments