
Staburo at the BIO Europe Conference 2016 in Cologne
 Staburo took part in the 2016 BIO Europe Conference in Cologne between 7 and 9 November.
Staburo took part in the 2016 BIO Europe Conference in Cologne between 7 and 9 November.
The conference featured a wide range of interesting topics for statisticians in the pharmaceutical and healthcare industry, including health technology assessment, biomarkers in translational medicine and general trends in drug development. Presenters from industry, academia and regulatory agencies shared their knowledge in talks and discussion rounds.
The most valuable feature of this conference was once again the partneringONE section, where we took the opportunity to meet potential partners face-to-face and learn about other their need in biostatistics. We will definitely be in Berlin for next years BIO Europe!

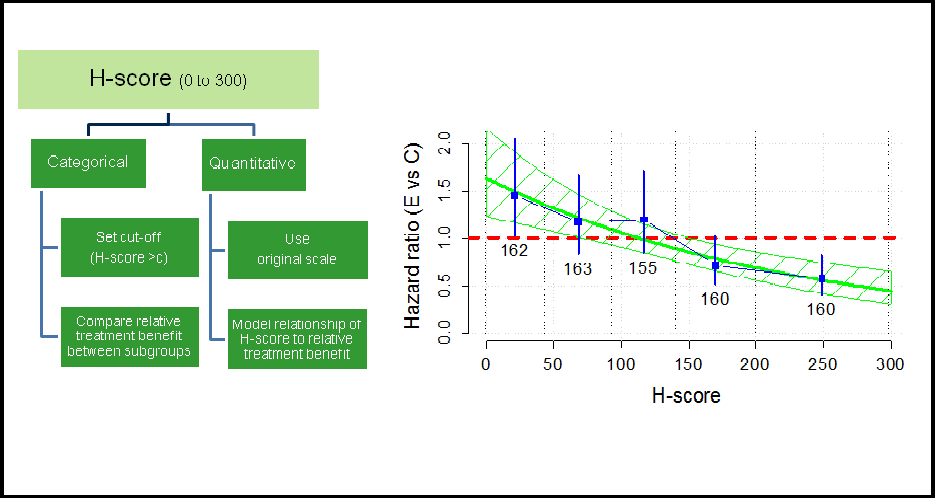
 The incorporation of biomarkers into drug development becomes more and more important, as they provide essential information to find the right therapy at the right time for the right patient (“precision medicine”).
The incorporation of biomarkers into drug development becomes more and more important, as they provide essential information to find the right therapy at the right time for the right patient (“precision medicine”).
 On 13 October 2016, managing directors and press officers of biotech, pharmaceutical and venture capital companies, as well as journalists, were meeting at the Faculty Club G2B of the IZB in Martinsried near Munich. There were three compact presentations held before a networking lunch:
On 13 October 2016, managing directors and press officers of biotech, pharmaceutical and venture capital companies, as well as journalists, were meeting at the Faculty Club G2B of the IZB in Martinsried near Munich. There were three compact presentations held before a networking lunch:
 We are very glad to welcome Vicky Stahl and Maximilian Siebold back in our team. Vicky and Max already worked as student interns at Staburo earlier and will support our clients in the area of statistical programming. We are very happy to have you back in the team!
We are very glad to welcome Vicky Stahl and Maximilian Siebold back in our team. Vicky and Max already worked as student interns at Staburo earlier and will support our clients in the area of statistical programming. We are very happy to have you back in the team!
 The statistical principles of equivalence trials and non-inferiority, as well as the special case bioequivalence, were presented. From a statistical perspective, being non-inferior or bioequivalent means to be only irrelevantly inferior or different, respectively. Based on this idea, the null hypotheses for non-inferiority or bioequivalence are shown.
The statistical principles of equivalence trials and non-inferiority, as well as the special case bioequivalence, were presented. From a statistical perspective, being non-inferior or bioequivalent means to be only irrelevantly inferior or different, respectively. Based on this idea, the null hypotheses for non-inferiority or bioequivalence are shown.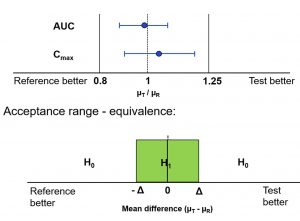
Recent Comments