
Cocktail course Christmas celebration
 This year’s Staburo Christmas party took place in the Barschule München, where we learned very much about famous Cocktails, its variants and how to produce them.
This year’s Staburo Christmas party took place in the Barschule München, where we learned very much about famous Cocktails, its variants and how to produce them.
We started the course with (single-)blinded sensorical testing of different alcoholic analytes, by smelling only. The best of us could get about half of the samples right, which showed a lot of training potential here.
Then, Matthias Knorr, the owner of the Barschule explained about Gin and Rum and we could – this time unblinded – taste the differences between the brands and production styles.
This was followed by a very active bottle juggling – or flair bartending – show in the training room of the school, where we could also practice some tricks.
In the last session, Matthias showed us how to produce tasty drinks – we even made a Lebkuchen (gingerbread) cocktail!


 Staburo took part in the APF workshop on 25 November 2016 in Berlin. This year, the APF has been invited by Parexel International GmbH.
Staburo took part in the APF workshop on 25 November 2016 in Berlin. This year, the APF has been invited by Parexel International GmbH.
 Staburo took part in this year’s BVMA symposium on 25 November 2016, at the Sheraton München Arabellapark Hotel, here in Munich.
Staburo took part in this year’s BVMA symposium on 25 November 2016, at the Sheraton München Arabellapark Hotel, here in Munich.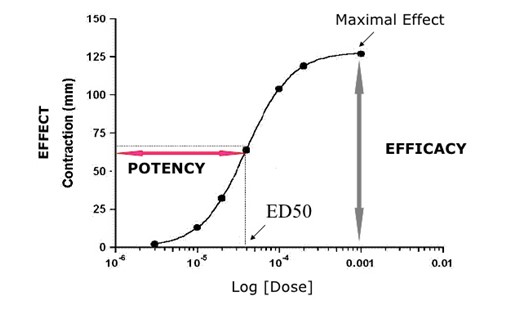
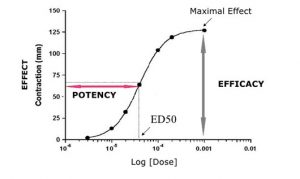 In this talk, important aspects of pharmacokinetics (PK) and pharmacodynamics (PD) were presented. Four elements of PK (ADME) were discussed in detail. Biopharmaceutical classification system (BCS), 2-compartment distribution model, destructive and conjugative reactions and the elimination phase of drugs were discussed for ADME trials. Some PK parameters were also listed and described. The second part of this talk was about pharmacodynamics, where the onset, duration and mechanism of actions were presented. In addition, some PD concepts such as ED50, efficacy and potency were discussed.
In this talk, important aspects of pharmacokinetics (PK) and pharmacodynamics (PD) were presented. Four elements of PK (ADME) were discussed in detail. Biopharmaceutical classification system (BCS), 2-compartment distribution model, destructive and conjugative reactions and the elimination phase of drugs were discussed for ADME trials. Some PK parameters were also listed and described. The second part of this talk was about pharmacodynamics, where the onset, duration and mechanism of actions were presented. In addition, some PD concepts such as ED50, efficacy and potency were discussed.
Recent Comments