News & Training
Staburo news about trainings, events and more
Staburo commented on EMA ‘Guideline on multiplicity issues in clinical trials’
Staburo submitted comments on EMA's draft guideline on multiplicity issues in clinical trials. Multiplicity issues arise in many areas of clinical development, including biosimilars and translational medicine. The guideline is intended to provide guidance on how to...
Staburo Strategy 2022 – Mission
Following our Strategy Workshop with Dr. Thilo Pfletschinger from 3DSE in May, we presented and discussed the results with all Staburo employees. Our mission statement is a short statement of our organization's purpose, identifying the scope of our operations. It...
Staburo’s statistical support in oncology biomarker study
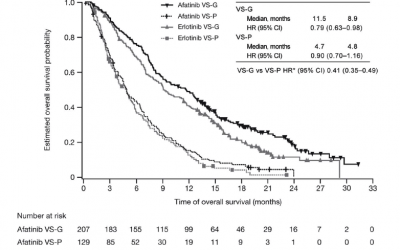
Staburo provided statistical support for a biomarker study that was recently published in the Journal Lung Cancer. The manuscript describes a retrospective analysis to assess the ability of the VeriStrat serum protein test to predict clinical benefit with afatinib...
2017 B2RUN Munich
The Staburo team once again proved that it has endurance, power and a great finish. This time, for a change, not in biostatistics projects - but in the 2017 B2RUN Munich, alongside 30,000 other runners. The race track was 6.1km long and took us through the picturesque...
Workshop Staburo 3DSE Biostatistics Strategy 2022
With the support of Dr. Thilo Pfletschinger from 3DSE, we developed the company strategy for Staburo for the next five years. Before the workshop, all employees gave input to possible goals and the prospects of Staburo. The final strategy includes all our common goals...
New member of the Staburo team
We are very happy to welcome Laura Schlieker in our team. Laura will support our clients with her experience in biostatistics, especially within the area of Translational Medicine & Biomarkers. We are looking forward to a great cooperation!
Training@Staburo: Guideline for sample size calculations at Staburo
The presentation was about the current guideline for sample size calculations that is in place at Staburo. This guideline has been presented to the whole team and is intended to help everybody, when performing such calculations in his/her projects. The first version...
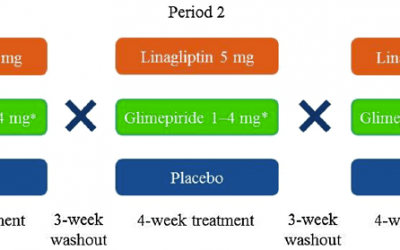
Staburo biostatistics support in diabetes trial
Another publication with Staburo biostatistics support, this time in diabetes type 2. Background: Studies of dipeptidyl peptidase (DPP)-4 inhibitors report heterogeneous effects on endothelial function in patients with type 2 diabetes (T2D). This study assessed the...
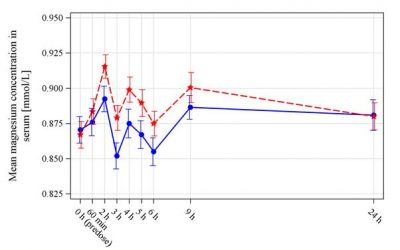
Publication of bioavailability study with biostatistics support of Staburo
Staburo delivered biostatistics services in this bioavailability study. The development of several disorders, such as cardiovascular diseases, diabetes and osteoporosis, has been linked to suboptimal dietary magnesium (Mg) intake. In this context, a number of studies...
You need support in data science?
Memberships and awards