Staburo Academy – Biostatistics and Data Science Trainings
Staburo is specialized in Biostatistics, Statistical Programming and Data Science. We cover a wide range of statistical services and develop tailor-made client solutions.
We love to share our statistical expertise and therefore we offer specific training programs. Our coaches are statisticians with a broad practical knowledge and teaching experience. The trainings can range from basic to very state-of-the-art statistical topics. All courses will be tailored to the specific interests of the audience, however our portfolio of existing courses can help to find the right combination of topics.

Clinical trial disclosure & transparency workshop
About the online course
There is an increasing demand for transparency in clinical trials. This interest in transparency is not only demanded by the public but also by global regulators like FDA (US) and EMA (EU), with the overall aim being to make as much clinical trial information public.
For study managers in smaller companies and in academia it can be hard to keep up with this constantly evolving landscape of regulations. Additionally, the scope of required information, the increased demand for disclosure of documents, and actual data entry into platforms is often challenging.
This interactive workshop is designed to bring you up to date with the currently applicable regulations. We will have group sessions to practice protocol and results data entry into the platforms and will give advice on handling timelines and necessary record updates. Following this workshop, you will be confident in disclosing your studies in full compliance with applicable regulations and your own transparency goals.
Topics
- Regulations in the US: FDAAA 801 and the Final Rule
- Regulations in the EU: CT Regulation 536/2014 (CTIS) and Clinical Trial Directive 2001/20 (EudraCT)
- Designing a ‘disclosure ready’ study
- Practical training on data entry into Clinicaltrials.gov and EudraCT/CTIS
- Document disclosure (redaction of PPD and CCI, deferral)
Target audience
The workshop is addressed (but not restricted) to industry or academia professionals who are interested in clinical trial disclosure and transparency.
General information
- Next course upon request
- 2 days: Both days will be from 13:00 to 17:00 local Munich time, online
- The detailed agenda will be sent out a few days before the start of the workshop
- The course will be held in English
- The fee includes the workshop slides and training material for the exercises
- A certificate of participation will be delivered at the end of the workshop
Fees
- The cost for this online course is 1100€ (applicable VAT will be added) for professionals from industry and academia
Agenda
Day 1:
- Overview of transparency processes during the lifecycle of a study
- FDAAA 801 and the Final Rule
- Posting results on ClinicalTrials.gov
- Registering studies and record maintenance on ClinicalTrials.gov
Day 2:
- Designing a ‘disclosure ready’ study
- EMA and CT Directive 2001/20 (EudraCT) vs. CT Regulation 536/2014 (CTIS)
- Posting results on EudraCT and/or CTIS
- Document disclosure according to the Clinical Trial Regulation
Registration
If you are interested in this online course, please contact us via e-mail academy@staburo.de
Further workshops
Staburo also offers tailor-made trainings in smaller groups or individual consultancy focused on your company’s needs.
For registration & for questions about the course
Estimands workshop
About the course
The estimand framework (ICH E9(R1)) is inherently collaborative.
Estimands shift the focus from statistical methods alone to a shared framework for designing, conducting, analyzing, and interpreting clinical trials, cross-functionally.
This is why our biostatisticians have partnered with regulatory medical writers. Together, they have designed a workshop tailored to your needs.
Topics and target audience
Three Levels
Level 1: The Big Picture
For business development, proposals, senior managers
How estimands support strategic planning
Duration: 1 hour
Focus:
- Explain the impact that estimands have on budgets
- Explain why timelines for protocol (and protocol amendment) development are impacted and where in the study’s lifecycle they will need to be extended
- Explain why unrealistically short timelines planned at the proposal stage could result in financial penalties if those timelines are not achieved
- Highlight implications for potential budget increases for later phase trials where there could be increases in the numbers of unique tables and future reporting implications
- Discuss the impact of estimands in trial registry postings
Participants will learn how estimands affect budgets and timelines, helping them plan strategically and save Sponsors time and money.
Level 2: Practical Utility
For medical writers & clinicians
How to plan estimands & shape documents
Duration: 2.5 hours
Focus:
- What is an Estimand?
- Presentation of estimands in the clinical study protocol (CSP)
- Leveraging estimands into trial design
- Reporting estimands in clinical study reports (CSRs)
- Case report form and informed consent form design considerations
- Reporting estimands in trial registry postings
Participants will learn how medical writers, clinicians, and statisticians work together to embed estimands into study planning from the start. The workshop will outline a practical process for developing estimands in the CSP and for reporting them clearly in the CSR and trial registries, aligned with the statistical analysis plan. Detailed statistical methods will not be covered.
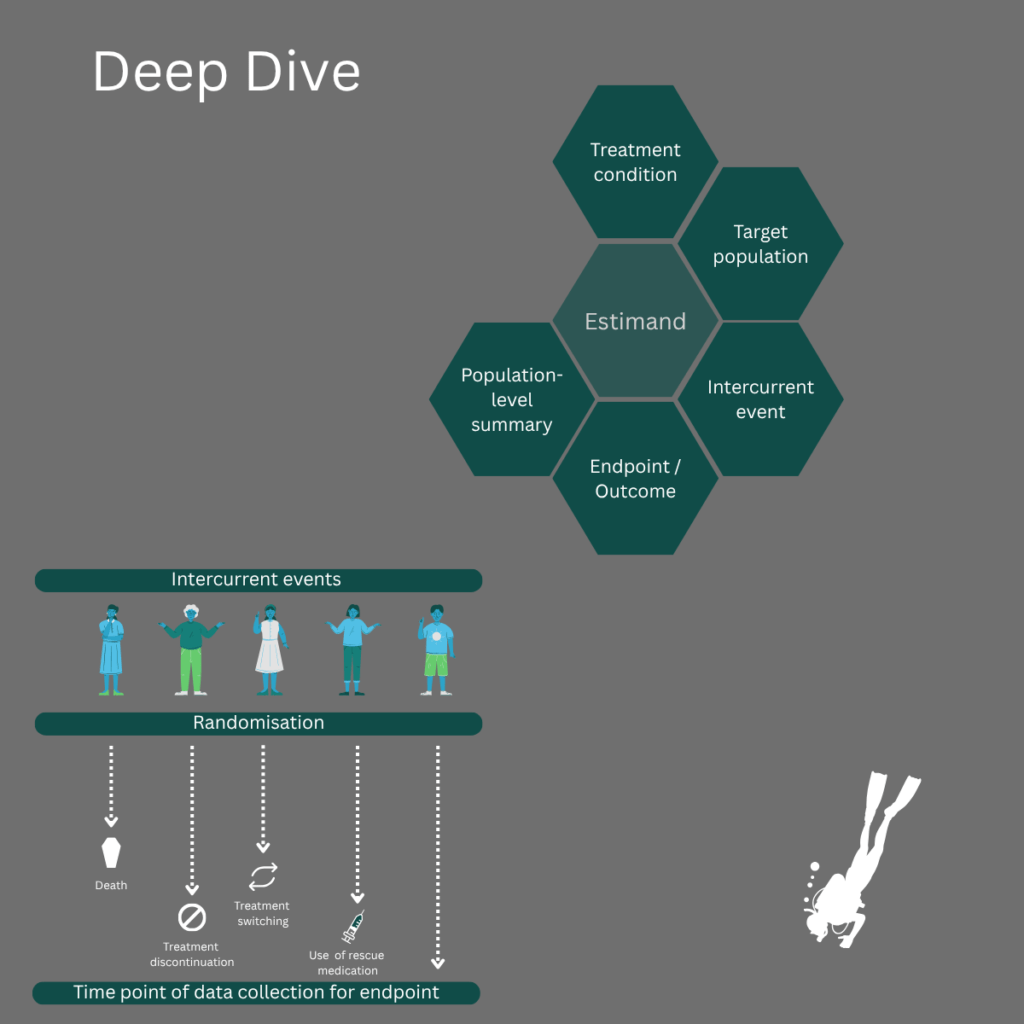
Level 3: Deep Dive
For statisticians & trial designers
How to align analysis with study intent
Duration: 2 days (approx. 6h each day including breaks)
Focus:
- Scope of ICH E9 (R1), the new estimands clinical development framework
- Concept of “intercurrent events” and strategies to address them
- Constructing an estimand (estimand attributes, interaction of all study stakeholders)
- Impact on trial design, conduct, and data collection
- Impact on trial analysis (missing data, sensitivity analysis)
- Incorporating estimands in the study protocol
- Experience from discussions with regulators
Participants will gain in-depth knowledge of the estimand framework. The workshop will use examples from different study phases and therapeutic areas to explore how to construct estimands, handle intercurrent events, address their impact on trial analyses (including missing data and sensitivity analyses), and more.
Each level includes practical and relevant exercises.
General information
- Language: English
- Time zone: CET / UK time
- Certificate of attendance: Yes
- Further tailored to your needs: available on request
**Details available on request**
Fees
- Fees of all 3 levels individually or combined: available on request
Instructors
Dr Gabriele Bleckert
Gabriele is our late-phase expert and a strong advocate of the estimand framework, both within the company and in the wider scientific community through her contributions to EFPIA’s Estimand Implementation Working Group (EIWG)-Reporting sub-team. She is the go-to person for complex questions on intercurrent events and a passionate mentor who builds bridges between clinicians, statisticians, and regulatory medical writers. Gabriele holds a PhD in Mathematics.

Dr Hannes Buchner
Hannes is our visionary Head of Biostatistics with deep expertise in estimands, particularly those related to treatment switching in oncology. Dedicated to advancing science and practice, he is well-connected across international statistical communities and actively fosters partnerships between academia and industry. An inspiring and supportive leader, he is committed to advancing the estimand framework and making it accessible to a broad audience. Hannes holds a PhD in Statistics.

Dr Sam Hamilton
Sam is one of our two regulatory medical writing experts with decades of industry experience, specialising in strategy and education. She is a past EMWA President and current Editorial Board Member of Medical Writing journal. Sam is known for demystifying estimands, inspiring cross-functional teams to embrace them, and for founding and chairing the influential CORE Reference Project. Sam holds a PhD in Gastrointestinal Physiology.

Dr Alison McIntosh
Alison is a regulatory medical writing expert and an experienced workshop leader with extensive industry experience across multiple global pharmaceutical companies. She makes estimands accessible by translating technical language into clear, practical guidance for non-statisticians, and contributes as a section editor for Medical Writing journal and a committee member of the EMWA CORE Reference Project. Alison holds a PhD in Molecular Virology from the University of Cambridge.

Next Estimands workshop
Want to finally make estimands work for your team?
BOOK YOUR SPOT!
Please email us at academy@staburo.de with the comment “Estimands” to get our info pack.
Staburo GmbH + Sam Hamilton & Alison McIntosh
For questions about the course
Introduction to Statistics course – 2 days
About the online course
The ability to understand and perform quantitative research is an essential skill in many fields. At the heart of quantitative research is statistical analysis, the ability to draw conclusion about a population based on a sample. This course will provide a solid foundation in statistics through practical application and with mathematical details kept to a minimum.
After this course you should be confident in understanding and performing quantitative analyses of numeric data. You will be able to incorporate quantitative analysis into high quality reports and output which enable decision making. This would include the ability to draw conclusions from data based on accurate statistical analysis.
Topics
- Descriptive Statistics: How to summarize data
- Exploration of Data
- Graphics and Presentation of Data
- Assessing Significant Differences Between Groups
- Investigation of Associations and Correlations
- Basic Trend Analysis
Target Audience
This course is designed for anyone wishing to understand basic statistical techniques thoroughly, e.g. : Clinical Research Associates, Project Managers, Team Managers, Principal Investigators, Medical Professionals, CMC Managers, Regulatory Affairs Managers.
General information
- The online course will be from 9:30 to 16:30 each day including breaks
- The detailed agenda will be sent out a few days before the start of the course
- The course will be held in English
- The fee includes the course slides and solutions of the exercises
- A certificate of participation will be delivered at the end of the course
Fees
- The cost for this 2-day online course is 1100€ (applicable VAT will be added) for professionals from industry and academia
Proposed agenda
Day 1: Variable Relationships, Visualizations & EDA
- Summarizing data
- Describing Multiple Variable Relationships
- Exploratory Data Analysis
Day 2: Probabilities, T-Tests, Correlations, Regression
- Probability and Inferential Statistics
- Comparing Categorical Variables
- T-Tests & Bivariate Plots and Correlations
- Introduction to Regression
Next Introduction to Statistics course
If you are interested in this course, please contact us at academy@staburo.de! Courses take place regularly, also on request.

For questions about the course
Introduction to R course – 2 days
About the online course
With over 2 million users worldwide R is one of the most popular open source programming languages in statistics and data sciences. As a preferred tool for complex data analyses it is applied in every industry. This R programming course will give an introduction to the basics of R. In the two-day course you will learn how to program in R, how to perform data analysis in R and how to visualize your results graphically.
Topics
Introduction to R
- Installation of R and R Studio (graphical user interface for R)
- Loading of packages
- Basic syntax in R
- Variable types and data structures, functions, operators
- Finding help to R functions
- Import and export of data
- Creating and editing R objects
- Working with data sets
Data analysis in R
- Basic descriptive statistics
- Linear regression
- ANOVA
- Hypothesis testing
Data visualization in R
- Creating and customizing basic plots
- Usage of high and low level plot functions
- Export of plots
Practice in R
- All topics will be provided with exercises in R
- The course will finish with a small project where the participants can apply all their gained knowledge about R
Target audience
The course is mainly addressed (but not restricted) to professionals who are interested in data science (e.g. Statisticians, SAS Programmers, Data Managers) and who want to learn the very basics of R. Further, this introduction is helpful for participants who wish to brush up on their knowledge in R.
Requirements
For this course prior knowledge in basic statistics or programming is helpful but not required.
General information
- The online course will be from 9:30 to 16:30 each day including breaks
- The detailed agenda will be sent out a few days before the start of the course
- Once you are registered a detailed explanation on how to install R and R Studio will be provided
- The course will be held in English
- The fee includes the course slides and solutions of the exercises
- A certificate of participation will be delivered at the end of the course
Fees
- The cost for this 2-day course is 1100€ (applicable VAT will be added) for professionals from industry and academia
Proposed agenda
Day 1
- Installation of R and R Studio (graphical user interface for R)
- Introduction to the software
- Loading of packages
- Basic syntax in R
- Variable types and data structures, functions, operators
- Finding help to R functions
- Import and export of data
- Creating and editing R objects
- Working with data sets
- Basic descriptive statistics
Day 2
- Linear regression
- ANOVA
- Hypothesis testing
- Creating and customizing basic plots
- Usage of high and low level plot functions
- Export of plots
- Small project in R applying the knowledge gained in the last two days
Next Introduction to R course
If you are interested in this course, please contact us at academy@staburo.de! Courses take place regularly, also on request.

For questions about the course
Advanced statistical methods course – 2 days
About the online course
This course is designed as a follow-up on our “Introduction to Statistics”-course. It gives you an overview on even more complex and advanced statistical methods, that are mainly used in the medical field.
By hearing this course, you will get an insight into a variety of advanced statistical methods ranging from generalized regression models like multivariable regression, logistic regression or mixed models to survival analysis and meta-analysis. In addition, these methods will be illustrated by real data applications in R.
Topics
Advanced / Generalized regression models
- Multivariable (linear) regression
- ANOVA
- Logistic regression
- Poisson regression
- Mixed models / GEEs
Survival analysis
- Survival data structure
- Kaplan-Meier estimator
- Log-rank test
- Cox proportional hazards model
Meta-analysis
- Idea / concept
- Statistical methods / forest plots
- Challenges and possible solutions
- Network meta-analysis
Target audience
This course is designed for advanced members having basic knowledge of statistics and programming skills in R.
General information
- The first day will be from 9:30 to 16:30 and the second day from 8:30 to 16:00
- The detailed agenda will be sent out a few days before the start of the course
- The course will be held in English
- The fee includes the course slides and solutions of the exercises
- A certificate of participation will be delivered at the end of the course
Fees
- The cost for this 2-day online course is 1100€ (applicable VAT will be added) for professionals from industry and academia
Proposed agenda
Day 1:
- Multivariable (linear) regression
- ANOVA
- Logistic regression
- Poisson regression
- Mixed models / GEEs
Day 2:
- Survival analysis
- Meta-analysis
Next Advanced statistical methods course
If you are interested in this course, please contact us at academy@staburo.de! Courses take place regularly, also on request.
For questions about the course
Machine learning and high-dimensional methods course – 2 days
About the online course
In the digital era where the collection of data is permanently increasing and phrases like “data mining” and “big data” are omnipresent, knowing how to turn the data into useful information is an essential skill. Therefore, basic knowledge of the most state-of-the-art machine learning techniques is an important instrument, not only for the ability to perform the appropriate statistical analyses in various settings, but also for a better understanding and interpreting of study results.
With this course you will get an insight into a variety of statistical methods for high-dimensional data ranging from parametric methods like PCA and LASSO to non-parametric machine learning methods like Random Forests, Boosting, SVMs and Neural Networks. In addition, these methods will be illustrated by real data applications in R.
Topics
- Introduction to high dimensionality and machine learning
- LASSO
- Principal component analysis
- Support vector machines
- K-means
- Decision Trees / Random Forests
- Boosting
- Neural Networks / AI
Target audience
This course is designed for advanced members having basic knowledge of statistics and programming skills in R.
General information
- The first day will be from 9:30 to 16:30 and the second day from 8:30 to 16:00
- The detailed agenda will be sent out a few days before the start of the course
- The course will be held in English
- The fee includes the course slides and solutions of the exercises
- A certificate of participation will be delivered at the end of the course
Fees
- The cost for this 2-day online course is 1100€ (applicable VAT will be added) for professionals from industry and academia
Proposed agenda
Day 1:
- Introduction to high dimensionality and machine learning
- LASSO
- Principal component analysis
- Support vector machines
Day 2:
- K-means
- Decision Trees / Random Forests
- Boosting
- Neural Networks / AI
Next Machine learning and high-dimensional methods course
If you are interested in this course, please contact us at academy@staburo.de! Courses take place regularly, also on request.
For questions about the course
